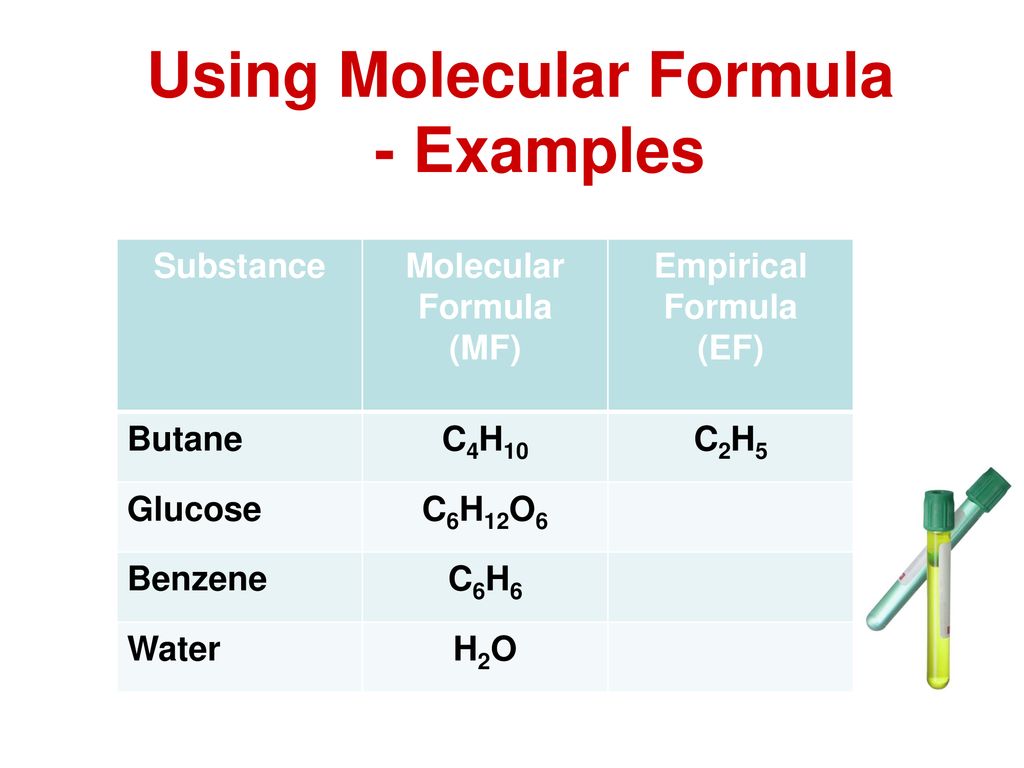
Empirical Formula of Butane
C_4H_10 then by dividing the subscripts by their HCF ie. What is its empirical formula.
Empirical Formula Of A Compound Ppt Download
By this we can consider that the empirical formula of butane is C_2H_5.

. Then you have 8266 g of C and 1734 g of H. Butane is a gas at room temperature and atmospheric pressure. This is the actual number of atoms of each element in a molecule of butane.
You can calculate the empirical formula by doing a combustion analysis. Butane is a highly flammable colorless. We can simplify the molecular formula C4 H10 which is the formula for butane by dividing the formula.
For every mole of carbon there are two moles of hydrogen. This gives you the empirical formula C₂H₅. This formula does not show the simplest whole number ratio because each number can be divided by 2.
Solution Assume 100 g of butane. Empirical formula of butane - 16921391 pankajkumarkhatuwala pankajkumarkhatuwala 27042020 Chemistry Secondary School answered Empirical formula. Of butane is C 4 H 10.
What is the empirical formula of butane. This is the actual number of atoms of each element in a molecule of butane. Highest common factor which is 2 in this case.
Was this answer helpful. Empirical formulas are the simplest form of notation. The carbon-to-hydrogen ratio equals 23.
If we know the molecular formula of butane ie. Hence the correct answer is C2H3. If you know that the molecular formula of butane is C₄H₁₀ then you divide the subscripts by their highest common factor 2.
Butane is 8266 C and 1734 by mass. The empirical formula of butane is C₂H₅. What is the empirical formula for butane eqC_4H_10 eq.
For every mole of carbon there are two moles of hydrogen. Butane is C 4H 10The emperical formula would be C 2H 5. The epirical formula for butane however is C2 H5.
The combustion of a sample of butane produces 16114 g of carbon dioxide and 08427 g of water. Moles of C 8266 g C. The molecular formula of butane is C 4 H 10.
This gives the empirical formula of butane - C 2 H 5. By using the expression Molecular formula n empirical formula. Hence the correct answer is C2H3.
C2H3 is the empirical formula for butane C4H6. What is the empirical formula for butane eqC_4H_10 eq. This is the actual number of atoms of each element in a molecule of butane.
1mol C1201g C 68826 mol C Moles of H 1734 g H. The formula for butane is C4 H10 because hydrocarbons have the formula Cn H2n2. The atomic mass of given empirical formula C 2H 12011 2100784.
The chemical formula represents the composition of the substance using the. 1mol H1008g H 17202 mol H. Empirical formula of butane can be calculated by two methods.
You burn a sample of butane and measure the masses of CO₂ and H₂O produced. This is the actual number of. The empirical formula of butane is CH.
0 The empirical formula for C4 H10 is C2 H5. The carbon-to-hydrogen ratio equals 23. C2H3 is the empirical formula for butane C4H6.
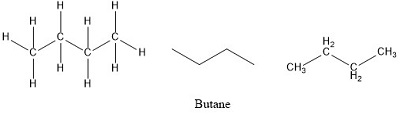
Butane or n-butane is an alkane with the formula C₄H₁₀. But the measured molecular mass for Butane is given as 581224u. Find a molecular formula for the compound having the empirical formula CH 2 with a molecular weight of 4208.

Butane Formula Structure What Is Butane Used For Video Lesson Transcript Study Com

Empirical Formula Flashcards Quizlet

Butane Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number Bond Pairs Lone Pairs Lewis Structure
Comments
Post a Comment